What I learned week 13
What
I learned week 13
The posted readings this week were
about brand name pharmaceuticals. I read the industry report as well as did a
little more research into the current landscape of the brand name
pharmaceutical industry, as well as what the future holds.
I
took my curiosity to the web and found a recent article, as recent as November
24th discussing how the Trump administration is taking on drug
prices. Trump wants to bring the hammer down on high drug prices, accusing
pharmaceutical companies of “getting away with murder.” What’s in discussion is
an idea to pass prescription drug rebates directly to seniors; like what
Medicare reimburses hospitals for certain drugs, it’s the same concept. It’s no
surprise that there are more and more generic drugs coming to market and
competition is high for brand name pharmaceuticals. “Last week, the agency
proposed a more than 700-page rule targeted at out-of-pocket drug costs. Among
the provisions, CMS sought feedback on the idea of providing rebates negotiated
between drug companies and their health plans directly to seniors buying drugs
under Medicare's part D prescription drug program. That would
increase premiums, but decrease the prices individuals face when filling
prescriptions (Johnson) .” After hearing Dr.
Kahn discuss Medicare Part D I was able to put everything into perspective.
Medicare Part D, also known as the Medicare prescription drug benefit program
is a government funded program to help subsidize the cost of prescription drugs
to Medicare beneficiaries. As I read more I realized that brand name pharmaceutical companies
should not receive the brunt of this act. In fact, the policies would affect
what people, or the government pay for drugs, but wouldn’t touch their prices
directly. This policy has triggered PBM’s who are the one’s who negotiate the
prices. “CMS, however, said in
its proposed rule that the current system
incentivizes drug plans to prefer high-priced drugs with large rebates over
cheaper drugs with smaller rebates — and added a change could better align the
interests of patients with those of the drug plan (Johnson) .” It’s clear
the Trump administration is tying to fight for the general public and lower
costs for patients, so it’ll be interesting to see how this plays out in the
future.
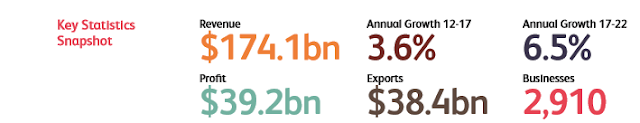
To paint a picture
of the current economy with brand name pharmaceutical manufacturing in the US,
here are some key statistics and numbers. Brand name pharmaceutical manufacturing
in 2017 gained a revenue of $174.1 billion dollars, and $39.2 billion of that was
straight profit. From 2012 to 2017, the industry grew at an annual growth rate
of 3.6%, and is expected to grow larger and faster by 2022 at a growth rate of
6.5%. The current top contenders in this industry include Amgen with 10.9%
market share, Johnson and Johnson at 10.4%, Merck and Co. Inc. at 9.6%, AbbVie;
9.5%, Pfizer Inc.;8.8% and Sanofi; 6.6% respectively. Some other important
facts to note are that the competition level, level of technology change,
globalization rates and barriers to entry are all very high; and the industry
life cycle stage is mature.
The article
started off by emphasizing an ongoing challenge for brand name pharmaceutical
manufacturing; and that is the concept of patent cliffs. Many blockbuster drugs
such as Lipitor or Zoloft face drug patent expirations. When this happens, many
low-price generic drugs come in and swamp the market. “As a result, many brand
name pharmaceutical manufacturers contended with intensifying competition from biosimilars[1]
produced by generic manufacturers, which cut into revenue growth (Oliver) .” Another issue that
brand name pharmaceutical manufacturing companies are facing comes from a push
by the government. The government has attempted to stimulate generic drug use
by setting favorable reimbursement rates for generic drugs. This has put a
strain on demand for brand name pharmaceuticals; in fact, “Generic drugs
account for 64.0% of Medicaid prescriptions, yet account for 18.0% of Medicaid
drug spending, according to US Pharmacist (Oliver) .” In response to
these challenges, brand name pharmaceutical manufacturing companies are
expanding their portfolios to include biotechnology, particularly biologic
drugs. From a science perspective, “Biologics
can be composed of sugars, proteins, or nucleic acids or complex combinations
of these substances, or may be living entities such as cells and tissues.
Biologics are isolated from a variety of natural sources - human, animal, or
microorganism - and may be produced by biotechnology methods and other
cutting-edge technologies (U.S. Food and Drug
Administration) .”
But from a business student perspective biologic are important to companies
because they typically benefit from greater patent protection; a.k.a. more
money in the brand name pharmaceuticals company’s pockets. So, the take
away here is that even though brand name pharmaceutical manufacturing companies
are being put under high pressure from the government as well as biosimilar
companies, the industry is still expected to grow, and will continue to grow
over the years to 2022.
Other important takeaways
in this article referred to globalization as well as international trade,
imports, exports etc. It’s interesting to see how much brand name
pharmaceutical manufacturing in the US relies on other countries. Globalization
in this industry is high and the trend is increasing. “Over the five years to
2022, industry imports are expected to grow at an annualized rate of 3.8% to
$101.0 billion (Oliver) .” Other countries
such as China, Brazil, and India are continuing to introduce, low cost, brand
name pharmaceuticals into the US. Many brand name manufacturers take their
research and development to other countries for there is a lower cost
associated. “A study by the consulting firm of Bain & Company reported that
the cost of discovering, developing and launching a new drug increased to more
than $1.5 billion (Oliver) .” Not only are brand
name pharmaceutical manufacturing companies going across seas because it is
cheaper, there is also a richer talent pool of well-educated individuals,
According to RNCOS Industry Research Solutions, the number of clinical trials
conducted in India is expected to surge to 8.0% of the worldwide total by 2016
(latest data available) (Oliver) .” This is for many
reasons such as easier clinical trial recruitment, lower labor costs, and tax
incentives. It is no surprise that every major drug developer will move toward
establishing operations in India.
At the end of the
day, there is always going to be a need and a want for brand name
pharmaceuticals; it’s simple marketing, consumers want “the best,’ and if
companies continue to spend money on consumer-directed promotions, more and
more patients will ask their physicians about drugs due to advertising they’ve
seen on television. From a health insurance
perspective, patients typically choose more expensive brand name drugs when
they do not incur the full cost of the medication. In some cases, not all,
brand name pharmaceuticals even have a higher efficacy rate. The industry is
continuing to grow and while they face struggles, as long as they continue to
hold their patent exclusivities and emerge with groundbreaking, specialized, well
marketed drugs; the industry will continue to thrive.
Works Cited
Johnson, Carolyn Y. The Trump administration is
taking on drug prices — but not drug companies. 24 11 2017.
https://www.washingtonpost.com/news/wonk/wp/2017/11/24/the-trump-administration-is-taking-on-drug-prices-but-not-drug-companies/?utm_term=.6d783f016aba.
26 11 2017.
Oliver, Kelsey. "Brand Name Pharmaceutical
Manufacturing in the US." Article. 2017. IBISWorld.
U.S. Food and Drug Administration. What Are
"Biologics" Questions and Answers. 05 08 2015.
https://www.fda.gov/aboutfda/centersoffices/officeofmedicalproductsandtobacco/cber/ucm133077.htm.
20 09 2017.
[1] A biosimilar is a
biologic medical product which is almost an identical copy of an original
product that is manufactured by a different company.
Comments
Post a Comment